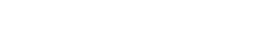3300
Company Profile
More than one hundred products are FDA-licensed, 40 products CE-marked
ISO 9001/ISO 13485/ISO 14001-certified|OEM package|10 automatic production lines
Manufacturing and Distributing Diagnostic Kits Worldwide
EGENS GROUP is a joint venture and a leading IVD solution provider. We develop, manufacture and distribute diagnostic kits worldwide, with manufacturing sites in Beijing, Nantong, and Wuxi, and products ranging from rapid test kits, Elisa kits, CLIA and POCT analyzer systems, with registered trademark as EGENS?.
Supported by 10 Automatic Production Lines
So far, EGENS has certified with QMS of ISO 9001, ISO 13485 CMDR, ISO 14001, OSHAS 18001, and GMP, with large production facilities of 19,500sqm class 10,000/100,000/200,000/300,000 clean rooms, 10 automatic production lines. More than one hundred products are FDA-licensed and 7 products FDA- cleared, and over 40 products CE-marked, which are exported to more than 106 countries and regions around the world.
2,700sqm Laboratory
EGENS sets up an R&D institute with South-East University, committed to providing state-of-the-art new products to the market, with a total of 2,700sqm labs with comprehensive lab equipment, mainly developing IVD reagents, Nano antibodies, diagnostic equipment, recombined antigen, monoclonal antibody, and more. EGENS also sets up the Nanobody (Nb) Bio-engineering Post-Doctoral Research Center, covering more than 2,000sqm, mainly developing diagnostic reagents and antibodies, which have been approved to be the technical engineering center of Jiangsu Province.
Get Prompt Reply Today
To know more about our products, please call us.